Which of the Following Describes a Solution Containing an Electrolyte
Electrolyte solutions - solutions that contain ions and therefore conduct electricity. Weak electrolyte solution.
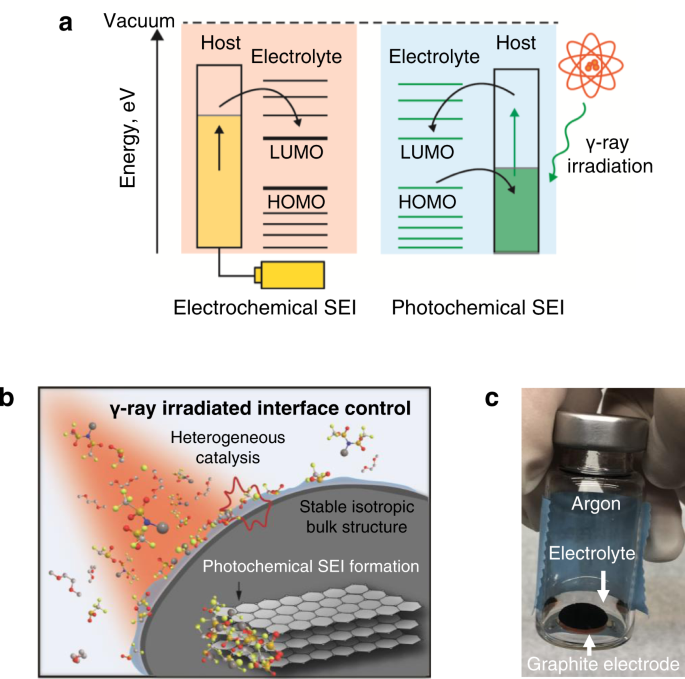
Photochemically Driven Solid Electrolyte Interphase For Extremely Fast Charging Lithium Ion Batteries Nature Communications
An electrolyte in a solution may be described as concentrated if it has a high concentration of ions or dilute if it has a low concentration.

. It is unstable and all the solute will precipitate if the solution is disturbed. Which of the following describes a solution containing an electrolyte. PbCl2 2 NaOH PbOH2 2 NaCl What would be the major species observed floating in solution after one mole of PbCl2 and 2 moles of NaOH are mixed in aqueous solution.
BVigorously shake a mixture of corn oil and water. All of the above are true concerning the sodium. To write the complete ionic equation.
Select the correct answer below O It typically contains a dilute solution. What is the molarity of a solution containing 70 moles of solute in 569 ml of solution What is the molarity of a solution that contains 6 moles of solute in 2 liters of solution The poh of a solution is 1075. It has a medium level of conductivity.
CDissolve a pinch of corn starch in a glass of water. C although sulfuric acid is a strong electrolyte an aqueous solution of H2S04 contains more HS04 ions than S04. The sodium is the solution B.
It cannot contain any more solute particles. Depending on the type of battery it can be a liquid or paste-like substance. It is unstable and all the solute will precipitate if the solution is disturbed.
It has the highest conductivity. An aqueous solution contains a weak electrolyte - An acid undergoes partial ionization in water - An aqueous solution contains a weak electrolyte Which of the following reactions is readily reversible and reaches chemical equilibrium under ordinary conditions. ADissolve a teaspoon of salt in a glass of water.
AH2SO4 bCuS cHF dZnNO32 Which are strong weak or non electrolytes. A solution containing more than the maximum amount of solute. O weak electrolyte o strong electrolyte o nonelectrolyte 8.
What is the initial concentration if a solution was diluted four 4 times with an aliquot of 500 mL and a total volume of 100 mL for each dilution. Gatorade as an electrolyte solution The sports drink Gatorade advertises that it contains electrolytes because it contains sodium potassium magnesium and other ions. Which of the following statements is true concerning the sodium.
If a high proportion of the solute dissociates to form free ions the electrolyte is strong. It is unstable and all the solute will precipitate if the solution is disturbed. The vant Hoff factor is.
Answer choices The solute particles are so firmly bonded that they do not break apart. Which of the following describes a solution containing an electrolyte. Answer choices The solute particles are so firmly bonded that they do not break apart.
From this observation you can conclude the solution is probably. The solute particles permit the passage of an electric current. It is highly reactive.
When humans sweat we lose ions necessary for vital bodily functions. O It contains electrolytes. When the ends of two wires from a circuit containing a battery and a lightbulb are placed into a beaker containing an aqueous solution the light bulb glows brightly.
It cannot contain any more solute particles. The solute particles permit the passage of an electric current. A NH3 contains no OH ions and yet its aqueous solutions are basic.
Which of the following describes the salt bridge solution in a standard galvanic cell. The battery electrolyte is a solution inside batteries. However no matter the type of battery the electrolyte serves the same purpose.
It cannot contain any more solute particles. The ionization of CH3COOH in aqueous solution Lis HNO3Aq to LiNO32aq H2g. It contains a completely dissociated solute.
A concentrated but weak base. Consider the following reaction. If most of the solute does not dissociate the electrolyte is.
That the solution. It contains a partially dissociated solute. Which of the following describes a way to make an electrolyte solution.
Which of the following solutions of strong electrolytes contains the largest number of moles of chloride ions. B HF is called a weak acid and yet it is very reactive. Answer choices The solute particles are so firmly bonded that they do not break apart.
DVigorously shake a pinch of table sugar in warm water. It transports positively charged ions between the cathode and anode terminals. 432 Explain the following observations.
Answer choices The solute particles are so firmly bonded that they do not break apart. It is unstable and all the solute will precipitate if the solution is disturbed. Start with a balanced molecular equation.
O It contains an elemental metal. An IV solution contains the electrolyte sodium. Na ions Cl ions.
Used to describe the chemical reaction while also clearly indicating which of the reactants andor products exist primarily as ions in aqueous solution. The sodium is the solvent D. Which of the following describes a solution containing an electrolyte.
The solute particles permit the passage of an electric current. It contains a complete solute. Which of the following describes a solution containing an electrolyte.
O 80M o 80M o 10M. It cannot contain any more solute particles. In the human body electrolytes have.
What is the concentration of oh ions in the solution. 1000 mL of 030 M AlCl 3 500 mL of 060 M MgCl 2 or 2000 mL of 040 M NaCl. The solution is unstable and solute will separate from it readily.
Which of the following describes water. Select all that apply. Which of the following statements correctly describe melting and melting point.
The sodium is the solute C. The solute particles permit the passage of an electric current. To replenish them we need to consume more ions often in the form of an electrolyte solution.

Physical Chemistry Positive Or Negative Anode Cathode In Electrolytic Galvanic Cell Chemistry Stack Exch Electrochemistry Galvanic Cell Chemistry Classroom

Effect Of Dilution On Molar Conductivity Of Weak And Strong Electrolyte Electrochemistry Chemical Energy Chemistry

Oxidation Reduction Electrolytic Cells Experiment 1 Redox Reactions Oxidation Reduction
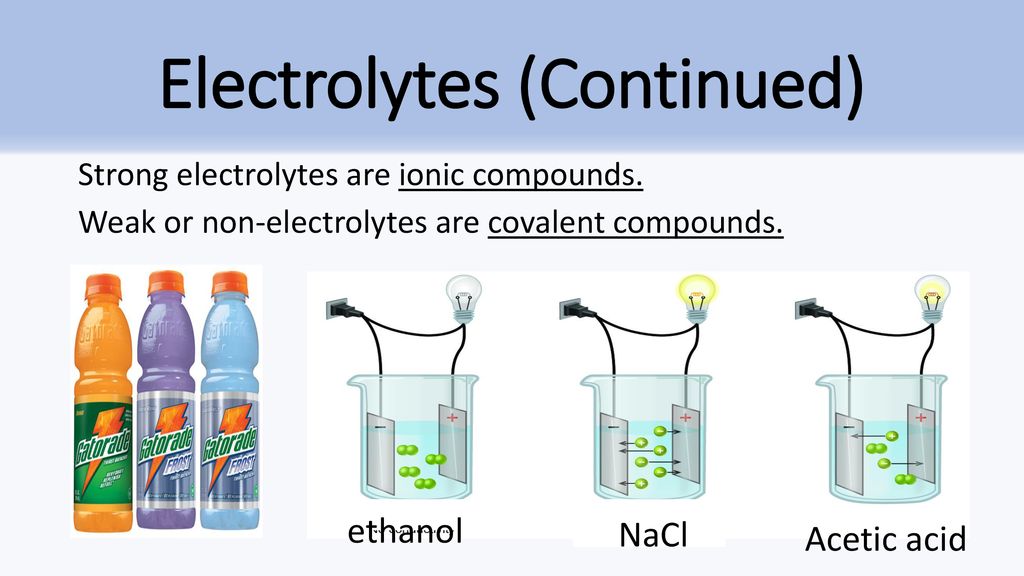
Solution Chemistry Ppt Download

Oxidation Reduction Electrolytic Cells 1 Oxidation Chemical Changes Redox Reactions

File Galvanic Cell Labeled Svg Galvanic Cell Chemistry Basics Teaching Chemistry

Salt Bridge Easy Science Electrochemistry Bridge Game Ap Chemistry

H2o The Mystery Art And Science Of Water The Chemistry Of Water Electrolysis In 2021 Chemistry Chemistry Education Renewable Energy Systems

Osmotic Pressure Easy Science Osmotic Pressure 10th Grade Science Study Skills

Everything In My Phone Gallery Phone Fuel Cells Gallery

Elektrochemische Zellen Elektrochemische Zellen Teaching Chemistry Electrochemistry Science Chemistry

Electrolysis Of Copper Sulphate Solution Download Scientific Diagram

What Is A Galvanic Cell Galvanic Cell Electrochemical Cell Science Clipart

Elektrokimia Sel Galvani Sel Volta Dan Sel Elektrolisis Soal Jawaban Galvanic Cell Chemistry Basics Chemistry Projects

Principle Of Electrolysis Of Copper Sulfate Electrolyte Electrical4u


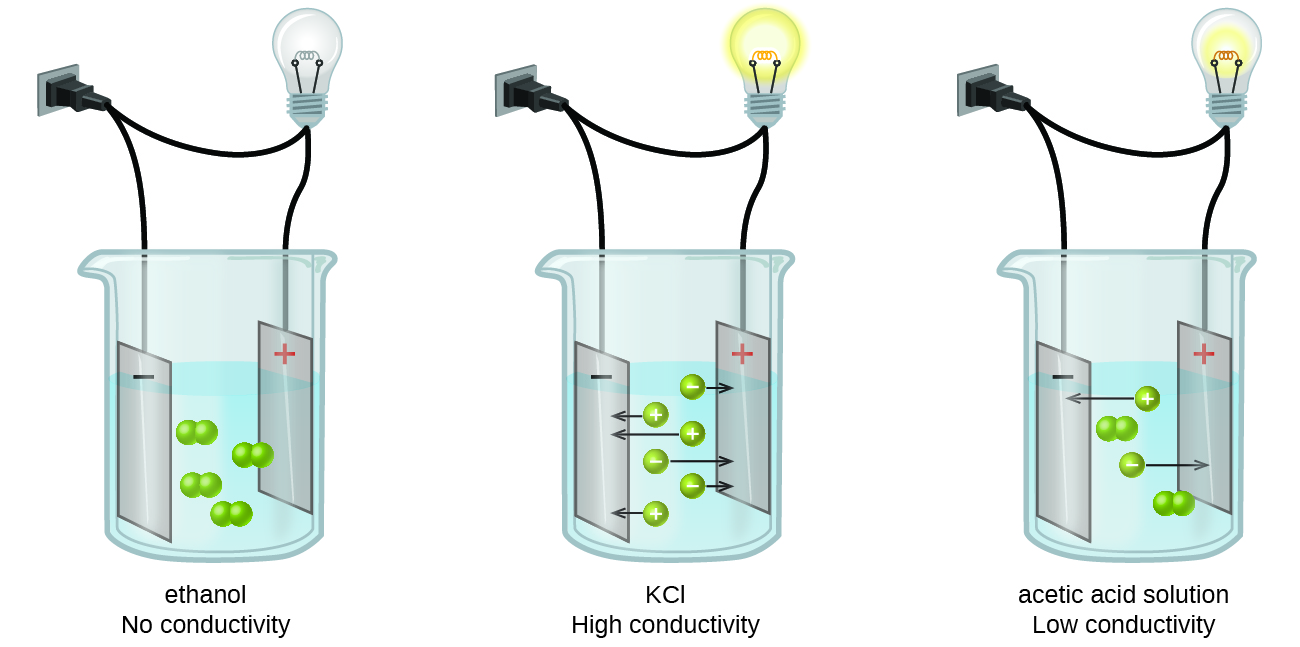

Comments
Post a Comment